Authors (9): S. Guan, P. R. Davies, E. K. Gibson, D. Lennon, G. E. Rossi, J. M. Winfield, J. Callison, P. P. Wells, D. J. Willock
Themes: Design, BAG DOI: 10.1039/c8fd00005k
Citations: 9
Pub type: article-journal
Pub year: 2018
Publisher: Royal Society of Chemistry (RSC)
Issue:
License: http://creativecommons.org/licenses/by/3.0/
Publication date(s): 2018 (online)
Pages: 67-85
Volume: 208 Issue:
Journal: Faraday Discussions
Link: http://pubs.rsc.org/en/content/articlepdf/2018/FD/C8FD00005K
URL: http://dx.doi.org/10.1039/C8FD00005K
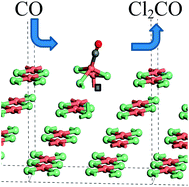
The interaction of CO with an attapulgite-supported Cu(II)Cl2 catalyst has been examined in a micro-reactor arrangement. CO exposure to the dried, as-received catalyst at elevated temperatures leads to the formation of CO2 as the only identifiable product. However, phosgene production can be induced by using a catalyst pre-treatment where the supported Cu(II)Cl2 sample is exposed to a diluted stream of chlorine. Subsequent CO exposure at ∼370 °C then leads to phosgene production. In order to investigate the origins of this atypical set of reaction characteristics, a series of X-ray absorption experiments were performed that were supplemented by DFT calculations. XANES measurements establish that at the elevated temperatures connected with phosgene formation, the catalyst is comprised of Cu+ and a small amount of Cu2+. Moreover, the data show that unique to the chlorine pre-treated sample, CO exposure at elevated temperature results in a short-lived oxidation of the copper. On the basis of calculated CO adsorption energies, DFT calculations indicate that a mixed Cu+/Cu2+ catalyst is required to support CO chemisorption.
| Name | Description | Publised |
|---|---|---|
| c8fd00005k1.pdf | Supl. data for Structural behaviour of copper chloride catalysts during ... | 2018 |
<< Previous Back Next >>