Authors (19): G. Qi, T. E. Davies, A. Nasrallah, M. A. Sainna, A. G. R. Howe, R. J. Lewis, M. G. Quesne, C. R. A. Catlow, D. J. Willock, Q. He, D. Bethell, M. J. Howard, B. A. Murrer, B. Harrison, C. J. Kiely, X. Zhao, F. Deng, J. Xu, G. J. Hutchings
Themes: New Catalysts DOI: 10.1038/s41929-021-00725-8
Citations: 199
Pub type: journal-article
Pub year: 2022
Publisher: Springer Science and Business Media LLC
Issue: 1
License: [{"start"=>{"date-parts"=>[[2022, 1, 6]], "date-time"=>"2022-01-06T00:00:00Z", "timestamp"=>1641427200000}, "content-version"=>"tdm", "delay-in-days"=>0, "URL"=>"https://www.springer.com/tdm"}, {"start"=>{"date-parts"=>[[2022, 1, 6]], "date-time"=>"2022-01-06T00:00:00Z", "timestamp"=>1641427200000}, "content-version"=>"vor", "delay-in-days"=>0, "URL"=>"https://www.springer.com/tdm"}]
Publication date(s): 2022/01/06 (online)
Pages: 45-54
Volume: 5 Issue: 1
Journal: Nature Catalysis
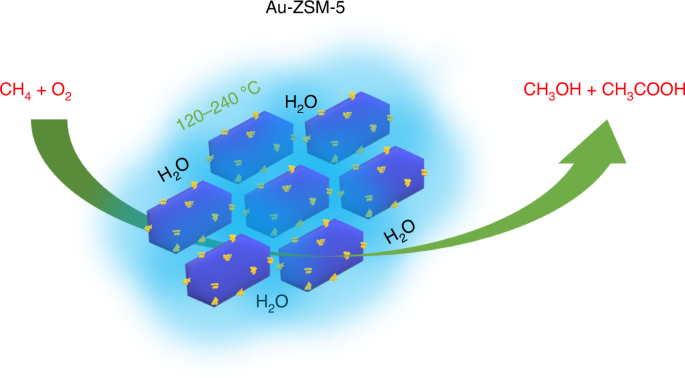
URL: http://dx.doi.org/10.1038/s41929-021-00725-8The oxidation of methane, the main component of natural gas, to selectively form oxygenated chemical feedstocks using molecular oxygen has been a long-standing grand challenge in catalysis. Here, using gold nanoparticles supported on the zeolite ZSM-5, we introduce a method to oxidize methane to methanol and acetic acid in water at temperatures between 120 and 240 °C using molecular oxygen in the absence of any added coreductant. Electron microscopy reveals that the catalyst does not contain gold atoms or clusters, but rather gold nanoparticles are the active component, while a mechanism involving surface adsorbed species is proposed in which methanol and acetic acid are formed via parallel pathways. The conversion of methane to oxygenated molecules is a very challenging reaction that often requires the use of a coreductant or stoichiometric conditions. Here, the authors report the use of gold supported on ZSM-5 as a promising catalyst for this process in combination with oxygen in the absence of coreductants.
| Name | Description | Publised |
|---|---|---|
| 41929_2021_725_MOESM2_ESM.xlsx | Source Data for Figure, statistical source data.... | 2022 |
| 41929_2021_725_MOESM3_ESM.xlsx | Source Data for Figure, statistical source data.... | 2022 |
| 41929_2021_725_MOESM4_ESM.xlsx | Source Data for Figure, statistical source data.... | 2022 |
<< Previous Back Next >>