Authors (13): E. L. Bell, R. Smithson, S. Kilbride, J. Foster, F. J. Hardy, S. Ramachandran, A. A. Tedstone, S. J. Haigh, A. A. Garforth, P. J. R. Day, C. W. Levy, M. P. Shaver, A. P. Green
Themes: Water-Energy DOI: 10.1038/s41929-022-00821-3
Citations: 360
Pub type: journal-article
Pub year: 2022
Publisher: Springer Science and Business Media LLC
Issue: 8
License: [{"start"=>{"date-parts"=>[[2022, 8, 11]], "date-time"=>"2022-08-11T00:00:00Z", "timestamp"=>1660176000000}, "content-version"=>"tdm", "delay-in-days"=>0, "URL"=>"https://www.springer.com/tdm"}, {"start"=>{"date-parts"=>[[2022, 8, 11]], "date-time"=>"2022-08-11T00:00:00Z", "timestamp"=>1660176000000}, "content-version"=>"vor", "delay-in-days"=>0, "URL"=>"https://www.springer.com/tdm"}]
Publication date(s): 2022/08/11 (online)
Pages: 673-681
Volume: 5 Issue: 8
Journal: Nature Catalysis
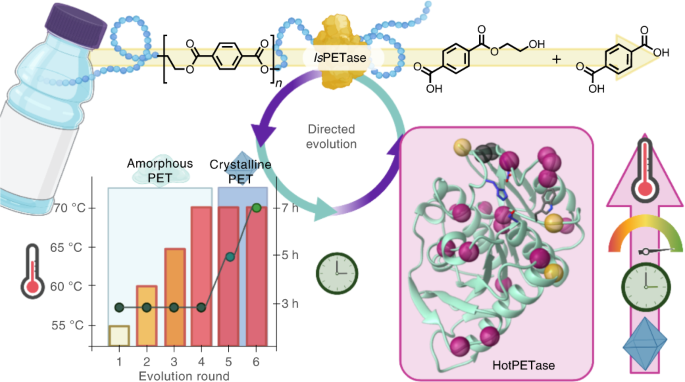
URL: http://dx.doi.org/10.1038/s41929-022-00821-3The recent discovery of IsPETase, a hydrolytic enzyme that can deconstruct poly(ethylene terephthalate) (PET), has sparked great interest in biocatalytic approaches to recycle plastics. Realization of commercial use will require the development of robust engineered enzymes that meet the demands of industrial processes. Although rationally engineered PETases have been described, enzymes that have been experimentally optimized via directed evolution have not previously been reported. Here, we describe an automated, high-throughput directed evolution platform for engineering polymer degrading enzymes. Applying catalytic activity at elevated temperatures as a primary selection pressure, a thermostable IsPETase variant (HotPETase, Tm = 82.5 °C) was engineered that can operate at the glass transition temperature of PET. HotPETase can depolymerize semicrystalline PET more rapidly than previously reported PETases and can selectively deconstruct the PET component of a laminated multimaterial. Structural analysis of HotPETase reveals interesting features that have emerged to improve thermotolerance and catalytic performance. Our study establishes laboratory evolution as a platform for engineering useful plastic degrading enzymes. Enzymes for poly(ethylene terephthalate) (PET) deconstruction are of interest for plastics recycling, but reports on their directed evolution are missing. Now, an automated, high-throughput directed evolution platform is described, affording HotPETase that effectively achieves depolymerization above the glass transition temperature of PET.
| Name | Description | Publised |
|---|---|---|
| PDB Entry - 7QVH The crystal structure of HotPETase, an evolved thermostable variant of IsPETase | PDB Entry - 7QVH(Status - Released) summary information: Title: The crys... | 2023 |
| 41929_2022_821_MOESM1_ESM.pdf | Supl. Methods, Figs. 1–23, Tables 1–4 and references.... | 2022 |
| 41929_2022_821_MOESM2_ESM.pdf | Reporting Summary... | 2022 |
<< Previous Back Next >>